Standing out is not just a matter of preference—it’s a necessity. It’s about challenging the status quo and finding novel solutions to long-standing problems. By encouraging unique ideas, we can collectively elevate the standard of care and make significant strides in healthcare innovation.
What Characteristics are most important in polyurethane foam components for medical device components?
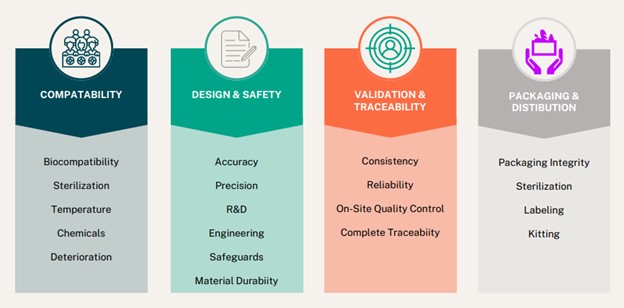
- Biocompatibility – Compatible with the human body to minimize the risk of adverse reactions. This includes considerations for allergic responses, tissue irritation, and cytotoxicity.
- Sterilization Compatibility – How will the device be sterilized and is polyurethane foam stable in that sterilization method?
- Reliability and Durability – Components should withstand normal wear and tear without compromising the device’s functionality.
- Accuracy and Precision – When precise measurements are critical, Technical polyurethane foam can be die-cut using milling, laser cutting, and digital cutting methods.
- Design – Even the larger companies we typically work with enjoy the collaboration of our R&D & Engineering professionals. We know foam, and we can suggest the best custom or non-custom foam for your device.
- Validation – Ensures the components consistently meet predetermined requirements and specifications. Guarantees reliability, functionality, and safety.
- Labeling and Instructions for Use – Clear and comprehensive labeling and instructions for use are vital for healthcare professionals and end-users. Proper labeling helps prevent errors in device usage and ensures that users understand the device’s intended purpose.
- Safety – Polyurethane foam can be engineered to safeguard medical devices exposed to electric or water hazards. This includes considerations for insulation, grounding, and absorption against potential safety issues.
- Materials Compatibility – What considerations for chemical compatibility, temperature, or resistance to deterioration are needed?
- Traceability and Quality Control – Traceability is crucial for tracking and identifying each device throughout its lifecycle. Quality control processes must be in place to ensure that each device meets established standards and specifications. Foamtec Medical is the world’s only vertically integrated polyurethane foam manufacturer and fabricator. Working with a single supplier and fabricator makes oversight easier.
- Packaging Integrity – Proper packaging is essential to protect the device during transportation and storage. Is sterile packaging or kitting required?
From the initial concept to the final product, our team of skilled scientists and engineers work meticulously with our customers, employing a highly collaborative approach. Medical-grade polyurethane foam is more than just what you can touch. As you’ve just read, there are many processes we can apply to differentiate your medical device in the market. By thoroughly understanding our customers’ specifications and expectations, we can deliver products that meet their exact requirements while exceeding your expectations.
Foamtec Medical will enter into a non-disclosure agreement with you to protect your intellectual property. We are an essential partner you can trust and rely on to keep your project on track. Share your feedback now!
Tell Us About Your Project
"*" indicates required fields

